| Size: | |
|---|---|
| Availability: | |
1:1 1:10 1:20
PUTH
Inactivation: It is mainly a virus lysis preservation solution modified from nucleic acid extraction lysis solution, added with lysis salt, but contains RNase inhibitors, which can protect the virus nucleic acid from degradation and enable subsequent detection by PCR.
Features: It is safer to use, can effectively prevent the operator from secondary infection, the operating
environment is not so strict, and can be stored at room temperature for a relatively long time, saving the cost of virus sample storage and transportation.
Non-inactivated type:
It is mainly a virus-maintaining liquid-type preservation solution that is improved on the basis of the transport medium, does not contain lysis salts, and is mainly based on Hank's solution, and is supplemented with such as gentamicin, fungal antibiotics, BSA (bovine serum) The fifth component of albumin), biological buffer HEPES, amino acids, cryoprotectants, glycerin and other components.
Features: The preserved virus has better integrity and higher detection rate, and can be used for other research besides nucleic acid detection. However, the requirements for the experiment are higher, and long-term storage after sampling needs to be kept strictly at low temperature.
Instructions:
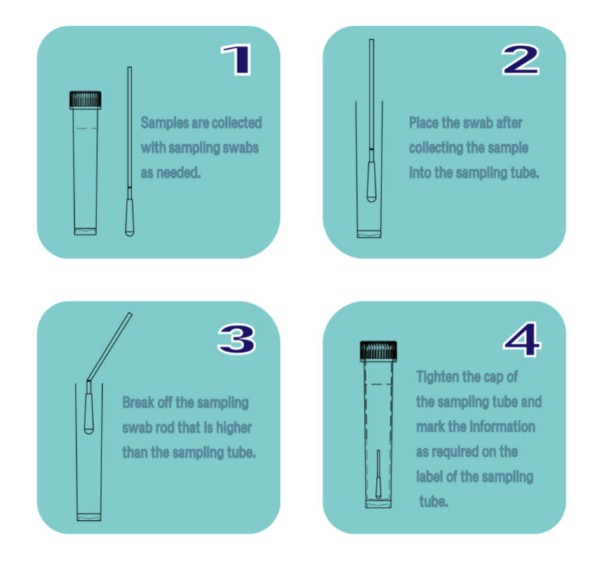
Company Introduction:
Chengdu PUTH Medical Plastics Packaging Co., Ltd, a wholly owned subsidiary of Wuliangye-Push Group, was established in 2006 with registered capital of RMB 350 Million, and located in Chengdu, one of the major economic center cities of China.
PUTH Medical is the first one-stop enterprise for PET tube in China, producing Blood Collection Tube from Raw Material to Finished-products.
and after over ten years' efforts, it has become a well-known company in the field of medical products in China, and is one of the largest Chinese manufacturers for medical plastic products.
Our Advantages:
PUTH MEDICAL imported machines and techniques from USA, Switzerland, Canada and Austria etc., dedicating to develop high qualified Medical Devices and Medical Package products.
PUTH MEDICAL has 1 invention patent, 19 utility model patents, and registered 14 trademarks.
PUTH MEDICAL workshops are built following the standards required by China GMP, EU CE and FDA;
Already certificated by ISO9001, ISO13485, CE, and China GMP (issued by CFDA).
PUTH MEDICAL invested RMB 10.9 Million for a 3200multifunctional Laboratory with 40 lab rooms; established Chemical testing center, Physics lab, Precision instrument rooms, and 120Bio-Performance testing room (class 10000 clean room, partial class 100), using most advanced detecting instruments and equipments.
PUTH MEDICAL workshops are built following the standards required by China GMP, EU CE and FDA; already certificated by ISO9001,ISO13485, CE, and China GMP (issued by CFDA).
PUTH MEDICAL workshops are built following the standards required by China GMP, EU CE and FDA; already certificated by ISO9001,ISO13485, CE, and China GMP (issued by CFDA).
Our Clean Workshop: